There are three laws of thermodynamics such as:
Zeroth Law of Thermodynamics: -
b) "The First Law states that energy cannot be created or destroyed". The amount of energy lost in a steady state process cannot be greater than the amount of energy gained. This is the statement of conservation of energy for a thermodynamic system.
The only difference here is that δW is the work done on the system. So, when the system (e.g. gas) expands the work done on the system is − PdV whereas in the previous formulation of the first law, the work done by the gas while expanding is PdV. In any case, both give the same result when written explicitly as:
And also the first law can be expressed as the Fundamental Thermodynamic Relation:
The entropy of a thermally isolated macroscopic system never decreases. However, a microscopic system may exhibit fluctuations of entropy opposite to that dictated by the Second Law.
- Zeroth Law of Thermodynamics
- First Law of Thermodynamics and
- Second Law of Thermodynamics
This Laws are discussed in detail, as follows
Zeroth Law of Thermodynamics: -
When two systems are each in thermal equilibrium with a third system, then two system are also in thermal equilibrium with each other.
A system is said to be in thermal equilibrium when its temperature does not change over time. Let A, B, and C be distinct thermodynamic systems or bodies. The zeroth law of thermodynamics can then be expressed as:
"If A and B are each in thermal equilibrium with C, A is also in thermal equilibrium with B."
The preceding sentence asserts that thermal equilibrium is a Euclidean relation between thermodynamic systems. If we also grant that all thermodynamic systems are (trivially) in thermal equilibrium with themselves, then thermal equilibrium is also a reflexive relation. Relations that are both reflexive and Euclidean are equivalence relations. One consequence of this reasoning is that thermal equilibrium is a transitive relation between the temperature T of A, B, and C:



- For example, if two systems of ideal gas are in equilibrium, then P1V1/N1 = P2V2/N2 where Pi is the pressure in the ith system, Vi is the volume, and Ni is the "amount" (in moles, or simply the number of atoms) of gas.
This law may be stated as follows:
a) "The heat and mechanical work are mutually convertible". According to this law, when a closed system undergoes a thermodynamic cycle, the net heat transfer is equal to the net work transfer.
a) "The heat and mechanical work are mutually convertible". According to this law, when a closed system undergoes a thermodynamic cycle, the net heat transfer is equal to the net work transfer.
b) "The First Law states that energy cannot be created or destroyed". The amount of energy lost in a steady state process cannot be greater than the amount of energy gained. This is the statement of conservation of energy for a thermodynamic system.
All laws of thermodynamics but the First are statistical and simply describe the tendencies of macroscopic systems. For microscopic systems with few particles, the variations in the parameters become larger than the parameters themselves, and the assumptions of thermodynamics become meaningless. The First Law, i.e. the law of conservation, has become the most secure of all basic laws of science.
The first law of thermodynamics basically states that a thermodynamic system can store or hold energy and that this internal energy is conserved. Heat is a process by which energy is added to a system from a high-temperature source, or lost to a low-temperature sink. In addition, energy may be lost by the system when it does mechanical work on its surroundings, or conversely, it may gain energy as a result of work done on it by its surroundings. The first law states that this energy is conserved: The change in the internal energy is equal to the amount added by heating minus the amount lost by doing work on the environment. The first law can be stated mathematically as:
where dU is a small increase in the internal energy of the system, δQ is a small amount of heat added to the system, and δW is a small amount of work done by the system.
The only difference here is that δW is the work done on the system. So, when the system (e.g. gas) expands the work done on the system is − PdV whereas in the previous formulation of the first law, the work done by the gas while expanding is PdV. In any case, both give the same result when written explicitly as:
And also the first law can be expressed as the Fundamental Thermodynamic Relation:
The law of conservation of energy states that the total amount of energy in an isolated system remains constant. A consequence of this law is that energy cannot be created or destroyed. The only thing that can happen with energy in an isolated system is that it can change form, that is to say for instance kinetic energy can become thermal energy. Because energy is associated with mass in the Einstein's theory of relativity, the conservation of energy also implies the conservation of mass in isolated systems (that is, the mass of a system cannot change, so long as energy is not permitted to enter or leave the system).
Second Law of Thermodynamics: -
Second Law of Thermodynamics: -
The entropy of an isolated system not in equilibrium will tend to increase over time, approaching a maximum value at equilibrium.
In a simple manner, the second law states that "energy systems have a tendency to increase their entropy" rather than decrease it. This can also be stated as "heat can spontaneously flow from a higher-temperature region to a lower-temperature region, but not the other way around." (Heat can flow from cold to hot, but not spontaneously—for example, a refrigerator requires electricity.)
A way of looking at the second law for non-scientists is to look at entropy as a measure of disorder. So, for example, a broken cup has less order than an intact one. Likewise, solid crystals, the most organized form of matter, have very low entropy values; and gases, which are highly disorganized, have high entropy values.
The entropy of a thermally isolated macroscopic system never decreases. However, a microscopic system may exhibit fluctuations of entropy opposite to that dictated by the Second Law.

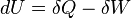
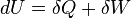
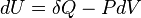
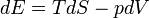
No comments:
Post a Comment